Oxidation and Reduction
Iron (II) and Iron (III)
T
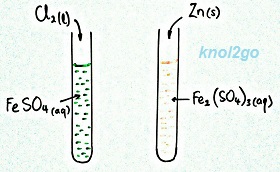
Test
tube 1
Fe2+(aq)
→
Fe3+(aq)
+ e-
Loss of Electrons = Oxidation
iron (II) is oxidised to iron (III)
Change in oxidation state: +2 to +3
Observation:
Green solution turns brown.
Chlorine Cl2 is the oxidising agent.
2e- + Cl2(l) → 2Cl-(aq)
Gain of Electron = Reduction
chlorine is reduced to chloride
Change in oxidation state: 0 to -1
Test tube 2
e- + Fe3+(aq) → Fe2+(aq)
Gain of Electrons = Reduction
iron (III) is reduced to iron (II)
Change in oxidation state: +3 to +2
Observation:
Brown solution turns green.
Zinc (Zn) is the reducing agent.
Zn(s) → Zn2+(aq) + 2e-
Loss of Electrons = Oxidation
Zinc is oxidised to Zn2+
Change in oxidation state: 0 to +2
Loss of Electrons = Oxidation
iron (II) is oxidised to iron (III)
Change in oxidation state: +2 to +3
Observation:
Green solution turns brown.
Chlorine Cl2 is the oxidising agent.
2e- + Cl2(l) → 2Cl-(aq)
Gain of Electron = Reduction
chlorine is reduced to chloride
Change in oxidation state: 0 to -1
Test tube 2
e- + Fe3+(aq) → Fe2+(aq)
Gain of Electrons = Reduction
iron (III) is reduced to iron (II)
Change in oxidation state: +3 to +2
Observation:
Brown solution turns green.
Zinc (Zn) is the reducing agent.
Zn(s) → Zn2+(aq) + 2e-
Loss of Electrons = Oxidation
Zinc is oxidised to Zn2+
Change in oxidation state: 0 to +2